How Medical Scan Anxiety May Be Impacting Pediatric Trial Recruitment
Medical imaging procedures — including MRI, CT, PET scans, and X-rays — are routine components of pediatric research protocols.
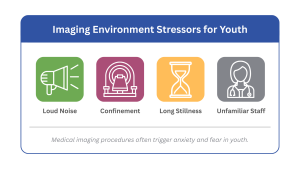
For children and adolescents, these procedures often involve:
- Loud environments
- Confinement
- Prolonged stillness
- Unfamiliar equipment
- Separation from caregivers
- Anticipation of discomfort
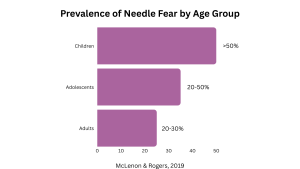
Anxiety and fear are common responses.1
For research teams, youth anxiety is not just emotional. When young people are not fully prepared or have high levels of anxiety about a part of the study protocol, it can impact the flow of activities which can, in turn, introduce procedural challenges for the research team and medical staff in the moment.
When youth experience imaging-related anxiety, they may resist procedures, move during scans, or require sedation. Families may hesitate about participation or follow-up imaging visits. Not only does youth fear impact participation rates, but movement during procedures can compromise scan quality, and sedation to reduce movement and anxiety introduces additional risk, cost, and complexity.
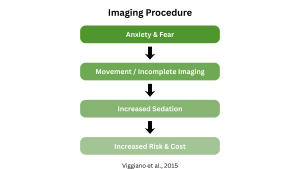
While participant anxiety around medical imaging procedures can potentially impact trial recruitment, retention, costs, and complexity, preparing participants can mitigate these risks. In fact, in pediatric MRI settings, youth psychological preparation significantly reduced their anxiety and fear and decreased the need for sedation by 18%.1
What does this mean for you? If imaging procedures are part of your protocol, youth emotional readiness may influence:
- Enrollment decisions
- Participant experience
- Scan compliance
- Procedural efficiency
- Overall study costs
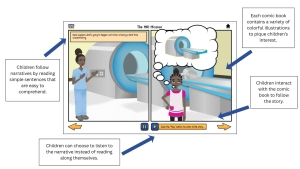
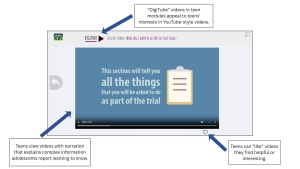
DigiKnowIt News prepares youth for research-related medical procedures through use of developmentally appropriate, interactive learning experiences that were designed to increase psychological readiness before participation decisions are made.

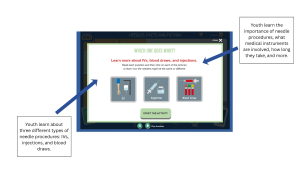
When customizing your DigiKnowIt News website, you can select web-based learning activities from a library of options to introduce children and adolescents to imaging procedures in an engaging, age-appropriate way.

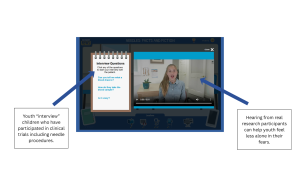
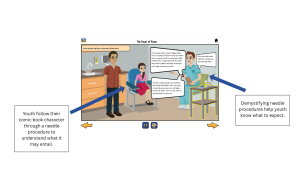
Unlike traditional methods of educating youth about imaging procedures and participation, DigiKnowIt News uses interactive content and illustrations to build understanding and confidence, helping reduce emotional barriers before scan day.

Increase your potential participants’ preparedness for your unique study. Explore and purchase DigiKnowIt News at digiknowit.com.
- Viggiano, M. P., Giganti, F., Rossi, A., Di Feo, D., Vagnoli, L., Calcagno, G., & Defilippi, C. (2015). Impact of psychological interventions on reducing anxiety, fear and the need for sedation in children undergoing magnetic resonance imaging. Pediatric Reports, 7, 5682. https://doi.org/10.4081/pr.2015.5682